Skyline share | Development of ultra-fine aluminum powder by evaporation in vacuum
The aluminum powder is produced by the atomization method, the average particle size is in the micron level, and it is difficult to meet the nano-level requirements for ultrafine powder. With the development of science and technology, the application of ultra-fine aluminum powder has become increasingly widespread. Such as, the solid propellant of rocket, the coating material of thermal spraying composite material, aluminum nitride, etc, it requires the particle size of aluminum powder to reach the standard of nanometer level.
 If aluminum is vaporized in a vacuum or inert gas environment, the molecular size of the gas is measured in angstroms (1Å = 10-10m). During the condensation process, the size of the crystal nucleus can be controlled to obtain nano-scale powder.
If aluminum is vaporized in a vacuum or inert gas environment, the molecular size of the gas is measured in angstroms (1Å = 10-10m). During the condensation process, the size of the crystal nucleus can be controlled to obtain nano-scale powder.
A model diagram of ultrafine particles prepared by gas evaporation method.
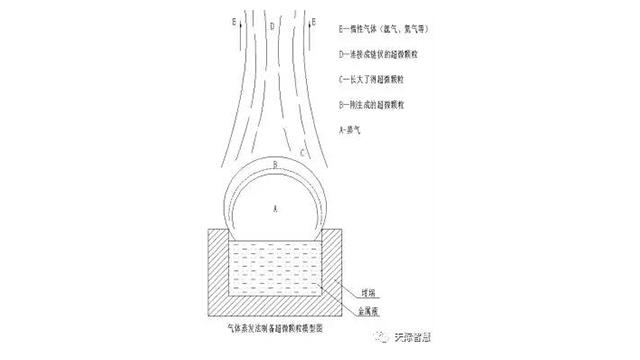 Gasification of aluminum
Gasification of aluminum
Aluminum has a melting point of 660.452 °C and a boiling point of 2520 °C. Aluminum is very easy to combine with oxygen at a higher temperature to release a lot of heat to generate AL2O3, so aluminum powder cannot be generated in an aerobic environment, and aluminum is evaporated in a vacuum or inert gas environment can be achieved.
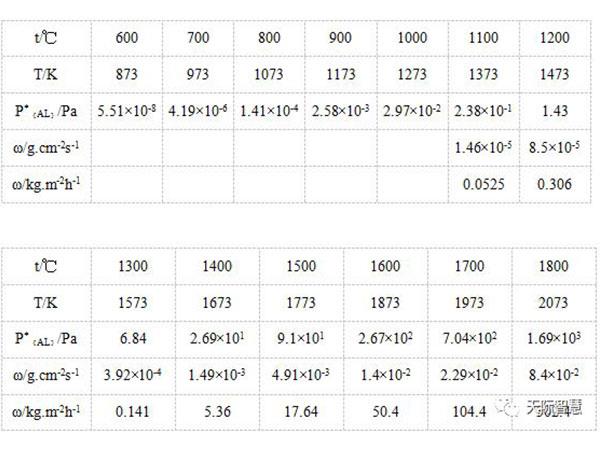
Saturated vapor pressure of pure aluminum:
LgP*{Al}/Pa=-16380T-1×lgT+14.445
Aluminum evaporation rate:
ω=0.0583×α×PTorr×
Data table of saturated vapor pressure (P), temperature (T) and ω of pure substance of aluminum:
 Aluminum vapor condenses into powder
Aluminum vapor condenses into powder
The preparation of ultrafine powder requires the condensation of aluminum vapor into a solid powder, which cannot be condensed into a liquid. The condensing temperature is set below the melting point of aluminum, increasing the degree of supercooling, forming uniform nucleation as far as possible, and falling to a cold place. The powder does not grow up during the landing process, and nano-level aluminum powder can be obtained.

A model diagram of ultrafine particles prepared by gas evaporation method.

Aluminum has a melting point of 660.452 °C and a boiling point of 2520 °C. Aluminum is very easy to combine with oxygen at a higher temperature to release a lot of heat to generate AL2O3, so aluminum powder cannot be generated in an aerobic environment, and aluminum is evaporated in a vacuum or inert gas environment can be achieved.
Saturated vapor pressure of pure aluminum:
LgP*{Al}/Pa=-16380T-1×lgT+14.445
Aluminum evaporation rate:
ω=0.0583×α×PTorr×
Data table of saturated vapor pressure (P), temperature (T) and ω of pure substance of aluminum:

The preparation of ultrafine powder requires the condensation of aluminum vapor into a solid powder, which cannot be condensed into a liquid. The condensing temperature is set below the melting point of aluminum, increasing the degree of supercooling, forming uniform nucleation as far as possible, and falling to a cold place. The powder does not grow up during the landing process, and nano-level aluminum powder can be obtained.

previous:Skyline share | The silicon-..
